
Objectives of the service
Microgravity for Personalized Medicine (M4PM) is a programme focused on 3D Tumour, Organoid and Spheroid growth in microgravity to enable the development of space-based personalised medicine. Set out under a partnership between Blue Horizon and Space Applications Services, and co-financed by the European Space Agency, the project aims to create both a technical model as well as a business model that replicates and enhances traditional terrestrial ones.
The way microgravity affects the behaviour and development of living organisms allows for research that cannot be conducted on Earth. The goal of the M4PM initiative is to evolve into an end-to-end service growing high quality spherical 3D Tumours, Organoids and Spheroids in low shear conditions in space, to better address some health and medical challenges.
These Space-grown Tumours, Organoids and Spheroids will be used for a wide range of applications and areas of research:
-
Drug testing: efficacy and toxicity
-
Drug development: (high throughput) screening of new substances
-
Disease modelling (incl. study of infectious diseases)
-
Modelling of ageing processes
-
Toxicity of substances
-
Personalized Medicine
-
Regenerative medicine
Users and their needs
The target user community are pharma and biotech companies, companies related to the chemical industry as well as academic research institutes globally.
It is generally recognized within the research community that the optimization and widespread implementation of 3D cell culture in vitro system will revolutionize the drug development & testing industry as a whole by replacing in vivo models as the gold standard. 3D organoids and spheroids are currently grown through the utilization of primary human tissue or pluripotent stem cells. These models exhibit multicellular self organisation and organ functionality, are capable of self-renewal, and can remain stable for extended periods of time during cultivation.
However, many of the obstacles facing the development of 3D cell culturing platforms for drug discovery & testing can be traced back to one issue: gravity. Using space to solve problems related to (personalized) medicine testing and discovery can be very effective not only with developing personalized (cancer) treatments, but also in increasing the rate of drug approval by using more relevant and realistic models than insufficient and inadequate (terrestrial) in vitro assays utilizing two-dimensional monolayers of (cancer) cells and in vivo animal models.
The user representatives involved in the project are originating from Pharma Industry, Biotech Industry (Organoid selling company) as well as a Cancer research institute.
Their needs are as follows:
-
High-quality TOS with homogeneous larger sizes then usually obtained under 1g conditions
-
Specific Space-phenotypes (e.g. aged phenotypes)
-
TOS with larger sizes but no necrotic cores
-
The flexibility to avoid or reduce the use of scaffolds or Matrigel for growth of TOS
These needs have to be met to optimize drug screening, drug testing, disease modelling, toxicity testing and personalized medicine using 3D cell constructs, TOS to be able to further reduce animal in vivo models and have better models reflecting the human body.
Service/ system concept
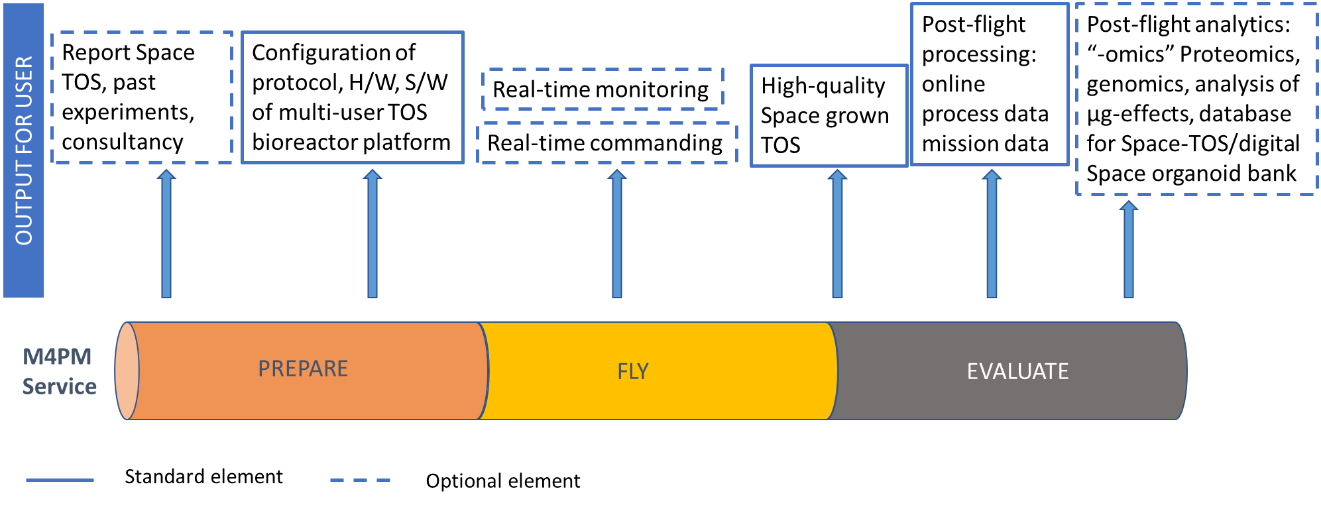
The overall service is consisting of 3 steps. Every step has standard elements as well as optional elements. The user is free to choose the optional elements whereas the standard elements are needed to have the service. The first step is “Prepare” where the multi-user generic TOS Bioreactor platform is customized by selection from a fixed amount of modules and scalable elements to fit the users needs. Moreover, the experiment protocol and software configuration are selected depending on the use case/user goal. Optionally the user might select the “report/review-service” elements which summarizes experiences of the specific TOS grown and tested in Space, thus enabling a better insight into µg effects for customers “new to Space”. The Fly element of the service is focussed on successfully bringing the experiment to Space and returning the “Product”, the space-grown TOS. Optional elements are real-time monitoring and real time commanding of and for certain online process parameters. The last step “Evaluate” consists of providing process data to the user and debriefing the mission together with the user. The user can choose post-flight analytics, which consists of further analytics, e.g. proteomics, genomics and a comparison with an in-house database of Space-organoid data from this field.
Initially the service will be offered as an end-to-end-mission service with basic package and optional customizations. (Pay for unit mission end-to-end service)
In a second phase M4PM will offer a Contract Research Organization service as known by pharma, with the simple exception of lab infrastructure being in space hence providing the identified premium. (Pay per batch protocol contracted research service fee)
In a third phase, we might consider for M4PM to also offer a Contract Manufacturing Organization service, also known and utilized by pharma, allowing to grow organoids or other 3D cell structures under the beneficial conditions of microgravity. (Pay per manufactured product).
Space Added Value
The Space-asset used for this service is the ISS, International Space Station and its microgravity environment.
Added values of space-grown tumours, organoids and spheroids:
-
High-quality tumours, organoids, spheroids with homogeneous larger sizes then usually obtained under 1g conditions
-
Specific Space-phenotypes (e.g. aged phenotypes)
-
Reduction of necrotic cores at the same sizes
-
The flexibility to avoid or reduce the use of scaffolds or Matrigel for growth of such organoids and spheroids

The added value of our Proposed Service as compared to other Space-associated providers:
-
offering a multi-user commercial generic TOS platform
-
offering optional elements to give more insight into the Space environment upstream and downstream to the user.
Current Status
M4PM ("Tumours, Organoids and Spheroids in Space - Microgravity for Personalized Medicine (M4PM)") has
undergone over the past 6 months a feasibility study around an end-to-end service growing high-quality
spherical 3D tumours, organoids and spheroids in Space. These Space-grown TOS are used for personalized medicine, disease modelling, drug research and (high-throughput) drug screening as well as toxicity testing.
Set out under a partnership between Blue Horizon and Space Applications Services, and co-financed by the
European Space Agency Business Applications, this first phase of the M4PM project addressed both the
technical aspects as well as a business model. For both technical and business aspects, the focus target was
the possibility to replicate and enhance traditional terrestrial models pharma & biotech users are accustomed
to. Throughout this feasibility study, the added value from space, the related value proposition, the service
definition and the technical setup that meets the needs of the user representatives have been addressed.
A M4PM community has been initiated with stakeholders (potential customers, partners, suppliers, CRO’s, life sciences clusters, incubators, …), comprised of Member companies and academics that currently grow and utilize tumours, organoids, spheroids in pursuit of (personalized) medicine (terrestrially). This M4PM community is being initiated to bring together thought leaders in industry and academia to solve problems and advance personalized medicine through innovations in microgravity.
Please refer to the M4PM website for more info: https://M4pM.space




