Objectives of the service
PASTA’s main objective is to use the viricidal and bactericidal capability of nonthermal plasmas to decontaminate and sanitise air without using filters or UV-C. The goal of the PASTA demonstration project is to evidence the potentials of the concept of a plasma-based air decontamination solution and quantify its decontamination capability against airborne pathogens.
Such a solution is designed to meet satisfy the requirements from NHS Hampshire hospitals and validate the prototype system performance under the operating conditions of NHS Hampshire hospitals
Users and their needs
More than 900,000 patients a year in England are infected while receiving medical treatment in hospitals. Recent studies demonstrate that airborne transmission is responsible for more than one third of hospital-acquired infections. It means that approx. 300,000 patients a year acquire airborne infection in NHS hospitals. The COVID-19 risk study [Nguyen et al] highlights that people who work in hospital settings had higher COVID-19 infection rate (20.2%) compared with that of the general population (14.4%). The direct user/beneficiary of the proposed solution, is the University Hospital Southampton NHS Foundation Trust as an early adopter, in which the PASTA system can be installed in areas less able to guarantee airflow rates and in waiting rooms. Building HVAC engineer and air filter suppliers, including Hamilton HVAC and Air Filtration Solutions, are targeted customers in the supply chain. The benefit of PASTA can also be extended to broader environments requiring safe clean air:
-
Dentistry and Operating theatres where appropriate in poorly ventilated dental surgeries/treatment rooms, but not likely to be beneficial in spaces with high ventilation rates.
-
Negative pressure rooms and areas including bronchoscopy rooms, sputum induction rooms, emergency room waiting area, airborne infection isolation rooms, and radiology waiting rooms
-
Private labs, buildings, and confined spaces such as cruise ship and public transportations where air quality can be quickly compromised.
Service/ system concept
The concept of the PASTA solution relies on the viricidal and bactericidal capability of non-thermal plasmas to decontaminate and sanitise air without using filters or UV-C. It is implemented into a portable platform so that the system can be easily deployed within the hospitals including poorly ventilated areas, clinical areas and waiting areas.
Preliminary biological test results show that more than a than 4-log reduction (99.99%) in pathogens can be achieved without using filters and fans, and that chemical compounds decompose. Therefore, the system can be applied to inactivating viral particles and reducing VOCs; eliminating the risk of the infection caused by viral/bacterial residues on filters; reducing the noise and discomfort caused by noisy fans.

Space Added Value
The PASTA uses two space technologies which are:
-
Space propulsion technology
Through ESA TDE (Cube-de-ALPS, 4000132060/20/NL/RA), a compact high-voltage pulse generator was developed as the power processing unit (PPU) of Cube-de-ALPS. This PPU was originally used as an ignitor of a vacuum arc thruster in the Cube-de-ALPS system.
-
Planetary protection and Life-supporting system technology
Through the UK Space Agency Exploration programme (UKSAG22_0006), porous plasma reactor through which air can pass had been designed. The developed porous plasma reactor can generate plasma-activated air and inject it rapidly into a water sample through a membrane system. Reactive oxygen species (ROS) in the plasma-activated air were used to inactive pathogens and decompose chemicals. It was demonstrated the system can inactivate 100% of E.coli K-12 and decompose 95% of chemical compounds in water samples.
Current Status
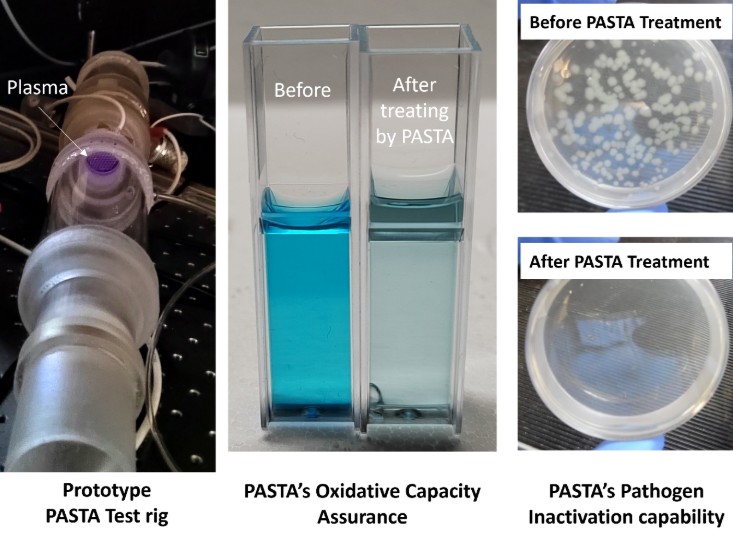
The Phase-1 PASTA project successfully demonstrated plasma-based air sterilisation, achieving over 2.5-log (99.7%) airborne pathogen reduction per unit. Biological tests confirmed 4-log (99.99%) inactivation of Pseudomonas aeruginosa, Enterococcus, and SARS-CoV-2, while oxidation capability tests showed 95% Methylene Blue degradation in 5 minutes, highlighting strong oxidative potential.
A PASTA test rig was designed with three key sections: pathogen injection and collection, plasma reactor, and post-treatment. The plasma reactor employs porous surface and volume Dielectric Barrier Discharge (DBD) reactors—volume DBD serves as the primary plasma source due to its longer lifespan, while surface DBD enhances sterilisation in high-risk areas. The post-treatment system ensures ozone levels remain below 0.01 ppm, meeting WHO safety standards.
Building on the success of Phase-1, Phase-2 aims to advance PASTA from a lab prototype to a scalable, deployable system, optimising performance, robustness, and regulatory compliance. A pilot deployment in Hampshire hospitals will validate real-world efficiency, paving the way for large-scale trials and commercialisation.