
Objectives of the service
Actinic Keratosis (AK) is the most common precancerous skin aliment. NICE estimates that AK affects 23% of the UK population aged 60 and over, 40% of Australians over 40, and 58 million Americans every year.
Photodynamic Therapy (PDT), thanks to its fast recovery time and improved cosmetic outcomes is the most preferred AK treatment by patients.
Currently, PDT treatment is done in two stages, all within a specialised clinic or hospital: the doctor applies a photosensitizing drug provided by a biotech company to the affected area and after an allotted time, the patient is treated using artificial light with a lamp or laser. This can be expensive and time consuming for all involved.
SatPDT is an innovative mobile telemedicine system designed to treat AK using clinically tested daylight Photodynamic Therapy (dPDT), removing the need for artificial lights.
This telemedicine digital technology opens the way for healthcare providers and biotech companies to offer safe and effective dPDT to their AK patients.
Users and their needs
SatPDT would allow patients to undergo an accurately monitored dPDT treatment at the hospital or, alternatively, a self-treatment at home using a mobile app. It also removes the need for hospitals to purchase expensive spectroradiometers and allocate specially trained staff to operate them.
Biotech manufacturers and Healthcare providers are the primary target audience and customers for SatPDT, with the end-users being clinical staff (e.g. doctors, nurses) or patients involved in the dPDT treatment of Actinic Keratosis.
Biotech providers need to be able to:
- Ensure that the software they provide is safe and accessible for home use
- Ensure their creams are compatible
- That the data is accurate.
Healthcare providers need to be able to:
- Adjust and monitor the time of PDT-effective daylight exposure and the dosage of the treatment cream
- Be confident that the treatment they are providing is working and that the data from the web-portal for managing the therapy sessions is accurate
- That the system provided is accessible to all the clinical staff involved
Patients need to be able to:
- Complete their PDT-effective daylight exposure
- Confident that their treatment is working
- That the system is easy to use and read
- Be confident that the data that is shared is confidential
The targeted users are in Europe (mainly UK, Spain, Germany, Italy), but the system can be extended all over the world.
Service/ system concept
SatPDT offers two key services:
- “AK dPDT Treatment Management”: a satellite-based digital system for the staff of hospitals & clinics aimed to support both the scheduling and the real- time monitoring of daylight PDT sessions of AK patients with an unprecedented level of accuracy;
- “Teledermatology Service”: a satellite-based mobile app for patients & caregivers enabling the home-based tele-medicine of AK through remotely supervised daylight PDT sessions anywhere, anytime.
The SatPDT system includes the following main IT architectural blocks:
- Geostationary Satellite Data Acquisition (GSDA)
- Environmental Data Processing (EDP)
- Skin Images AI Processing (SIAP)
- User Interfaces (UI)
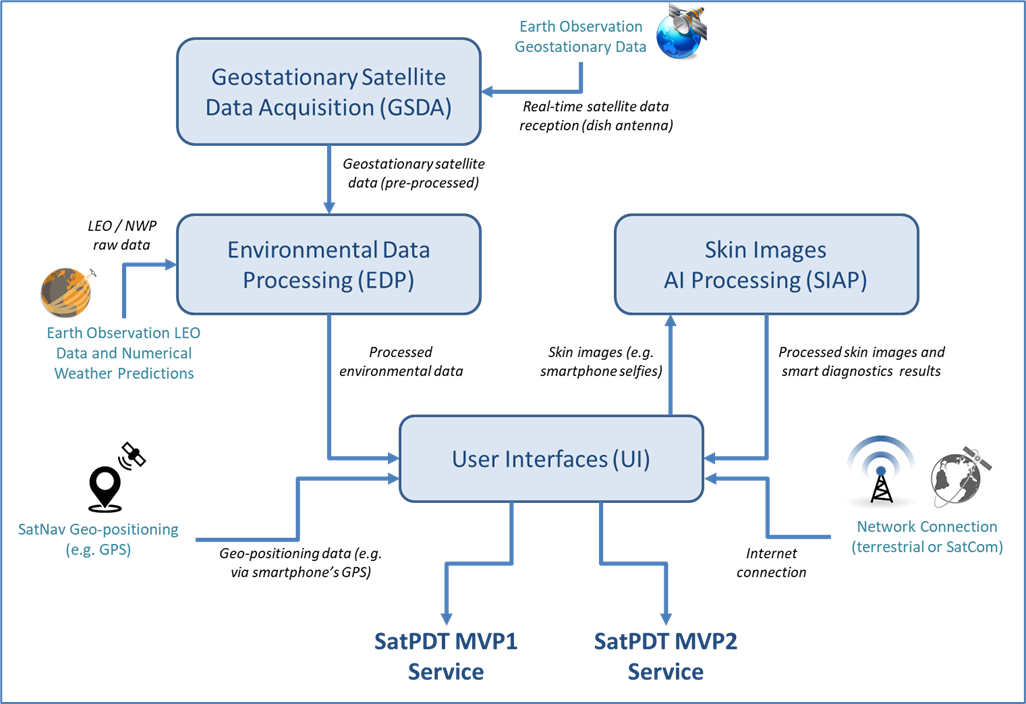
The GSDA block performs a real-time monitoring of atmospheric conditions all over the world in near real-time by continuously acquiring data from Earth Observation satellites. Then, the EDB block calculates the dPDT-effective solar irradiance dose incident in any location detected by SatNav satellites starting from these atmospheric data, supporting the delivery of dPDT sessions anywhere. The assessment of the efficacy of a dPDT session can be supported by the SIAP block, providing an Artificial Intelligence-based processing of smartphone photos of AK lesions post-treatment.
Finally, the UI block includes the web-portal for clinical staff and the iOS/Android mobile apps for patients/caregivers.
Space Added Value
The SatPDT services rely mainly on the following two space assets:
- Earth Observation;
- Satellite Navigation.
Indeed, the SatPDT services are based on the real-time monitoring & forecasting of dPDT-effective solar irradiance dose (“dosimetry”). This can be done with a sufficient level of accuracy only by using the SatPDT processing of Earth Observation satellites imagery coupled to a SatNav-detection of geo-location, unless a very expensive and periodically-calibrated spectroradiometer is available.
This is a key and unprecedented added value provided by SatPDT. Indeed, the current alternative technologies for dPDT-effective irradiance monitoring are definitely less convenient for clinicians and patients:
- spectroradiometers, slightly more accurate than the satellite-based method but extremely expensive and to be periodically calibrated (difficult to be maintained);
- portable radiometers, not reliable and not accurate enough (and not providing PDT-effective irradiance directly);
- online weather predictions, not accurate enough (and not providing PDT- effective irradiance, just cloudiness or UV Index to be then processed).
Data derived from McLellan et al. (2020), Photodiagnosis Photodyn Ther 31:101914.
Current Status
The project is currently at the end of the Feasibility Study phase. Several stakeholders have already demonstrated their interest for the project. In particular, some of them have also expressed in writing their interest to be involved in the potential follow-on Demonstration Project (envisaged to start in early 2021) and their commitment to directly support & host the 2 envisaged pilot demonstration campaigns (in the UK and in Spain):
- 2 multinational biotech companies, manufacturing dPDT pro-drugs to be potentially coupled to SatPDT services;
- 3 public hospitals delivering dPDT treatments.
So, after the technical & economic feasibility/viability assessment performed during the Feasibility Study, the next step is to actually develop, integrate and test the whole SatPDT system and its two services, for then demonstrating it operationally in the frame of the 2 pilot campaigns, paving the way for a future launch on the worldwide market.


