
Objectives of the service
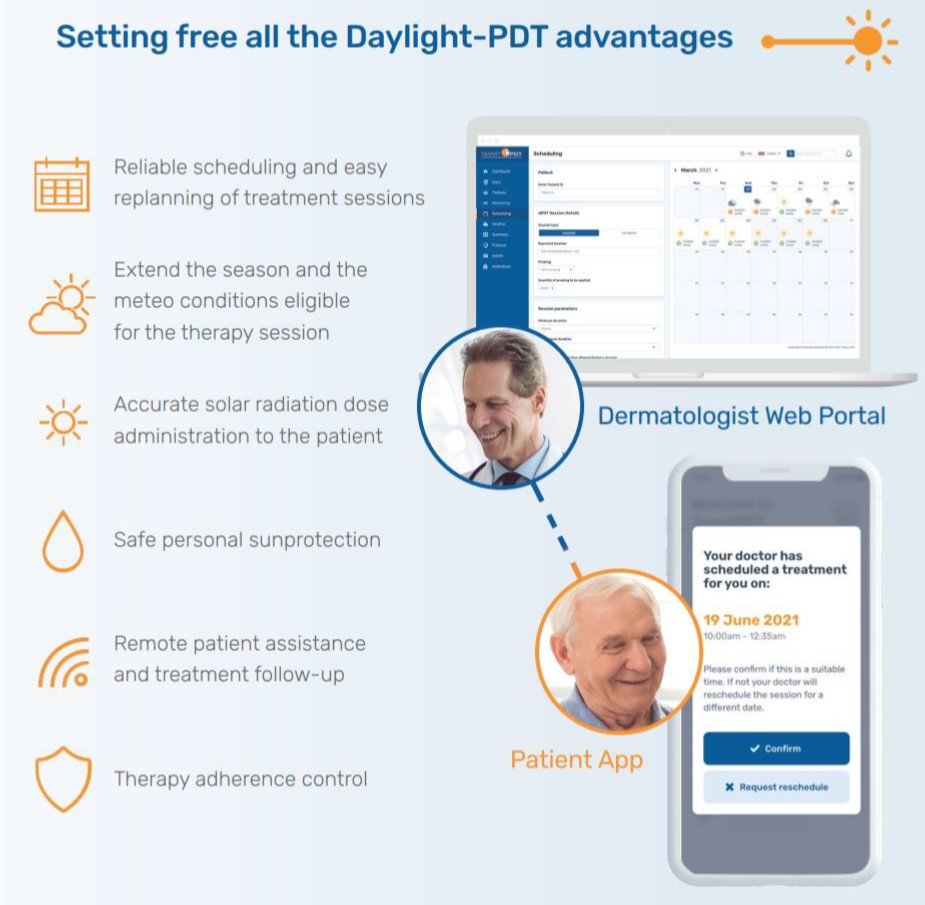
The SmartPDT product developed in the frame of SatPDT is a satellite-based innovative digital solution for the treatment of skin cancer and pre-cancer (e.g. AK). It facilitates the delivery of the dPDT therapy, saving costs and time for both patients and clinical staff as well as increasing its reliability by also including a follow-up remote support.
Indeed, the SatPDT system uses Earth Observation satellite data for monitoring in real-time the solar radiation dose received by a patient during a daylight therapy session, thanks also to the geo-localisation performed via satellite navigation systems (e.g. GPS sensor on patient’s smartphone). This can be performed anytime and anywhere, supporting the clinical staff in delivering from remote quick, easy, and effective dPDT treatments to a considerably larger number of patients.
Specifically, the SmartPDT solution will provide several benefits to clinical staff and to patients with respect to the current dPDT practice as reported in the table below.
| Benefits of SmartPDT with respect to current dPDT practice | Description |
| Daylight PDT (dPDT) digital enabler | SmartPDT is an integrated digital solution (not existing currently for dPDT), co-designed and tested by clinical staff and patients to create a friendly and inclusive solution, being usable for those who are both novice and well-trained in dPDT. |
| Home-based dPDT | SmartPDT will save patients the hassle of visiting hospitals and clinics for their Actinic Keratosis treatment, allowing them to schedule their dPDT treatment around their personal lives. |
| No need for dosimetry devices | SmartPDT is offered as a Software as a Service (SaaS), so there is no need to install any hardware or radiation sensors. |
| Time saving for clinical staff | SmartPDT provides easy and effective management of the dPDT protocols, providing to doctors and clinical staff the benefit of reduced administrative burden, saving treatment management time and increasing the number of patients undergoing treatment at the same time. |
| Easy dPDT scheduling | SmartPDT makes treatment scheduling for each patient easy by highlighting the most suitable treatment time slot by forecasting when it is best to receive the recommended solar radiation light dose. |
| Patient follow-up supervision | Effortlessly and remotely follow-up on patient treatment progress using SmartPDT with accurate treatment protocol reports to see how well patients have followed the therapy guidance. This will also include an AI-assisted analysis of patient Actinic Keratosis lesions within the SatPDT photo gallery for immediate therapy efficacy and adverse effects control. |
| Remote patient monitoring | SmartPDT enables dermatologists and clinicians to remotely monitor their patient’s progress, proactively adjust Actinic Keratosis treatment plans, supervise the patient behaviour and the AK lesions status during and after the therapy session. |
Users and their needs
The SmartPDT product developed in the frame of the SatPDT project is aimed to be directly used by:
-
Clinical teams delivering dPDT treatments (e.g. clinics or hospitals)
-
Patients undergoing a dPDT treatment, e.g. patients with Actinic Keratosis (AK).
Moreover, the biotech pharma companies manufacturing prodrugs for dPDT (cream or patches) are interested to be commercially involved, supporting an increased adoption of dPDT as preferred treatment for Actinic Keratosis (AK) or other skin cancer / pre-cancers thanks to the use of SmartPDT.
Indeed, currently there are no digital solutions for properly managing daylight PDT, there are no accessible and reliable methods for monitoring PDT-effective solar irradiance and there are no specific smart tools for AK diagnostics aimed to support the dPDT treatment follow-up and its remote supervision.
SmartPDT can address all these points in a unique and very innovative way, answering to the clear user needs (operational and market needs) expressed by the key Users involved in the SatPDT project:
-
Ninewells Hospital of Dundee, NHS Tayside (UK)
-
University Hospital Miguel Servet of Zaragoza (Spain)
-
NHS Oxford University Hospitals (UK)
-
Dr Richter-Hintz Clinic (Germany)
-
Biofrontera Gmbh (Germany)
-
Photonamic Gmbh (Germany)
-
Galderma Italia (Italy)
Service/ system concept
The SatPDT project aims to develop two different services, or modalities of use, via the siHealth’s SmartPDT product:
-
1. Hospital-based dPDT Treatment Management;
-
2. Home-based dPDT Teledermatology Service.
The first one is a hospital-based service, intended to support the planning and delivery of dPDT sessions for treating skin pre-cancer (Actinic Keratosis - AK) in the outdoor areas nearby hospitals and clinics. It will be provided to hospitals and clinics (the customers of this service) via a dedicated web-portal supporting the clinical staff both in scheduling and in real-time monitoring outdoor daylight treatments by means of solar irradiance data calculated from Earth Observation satellite imagery (PDT-effective solar irradiance). It will also include an Artificial Intelligence-based image processing component for supporting the dermatologists in skin pre-cancer lesions diagnostics by elaborating smartphone camera photos.
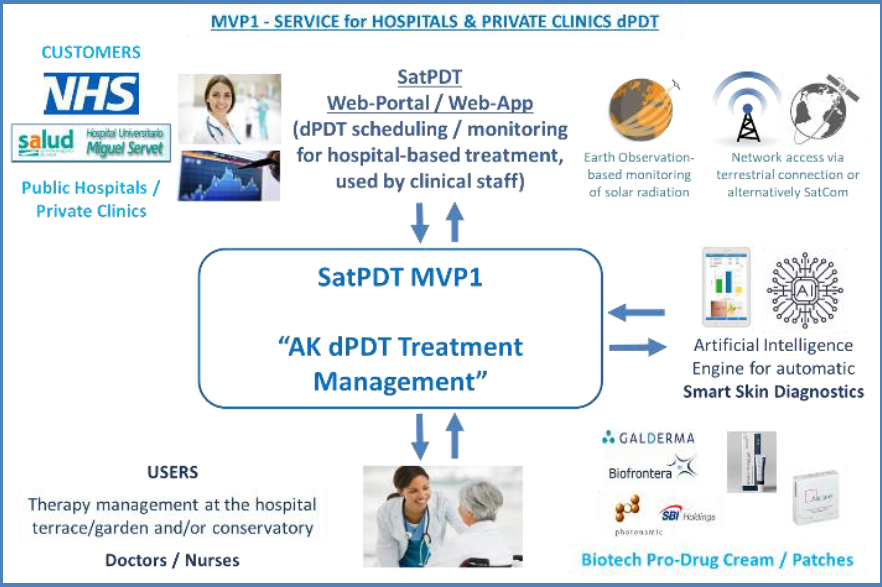
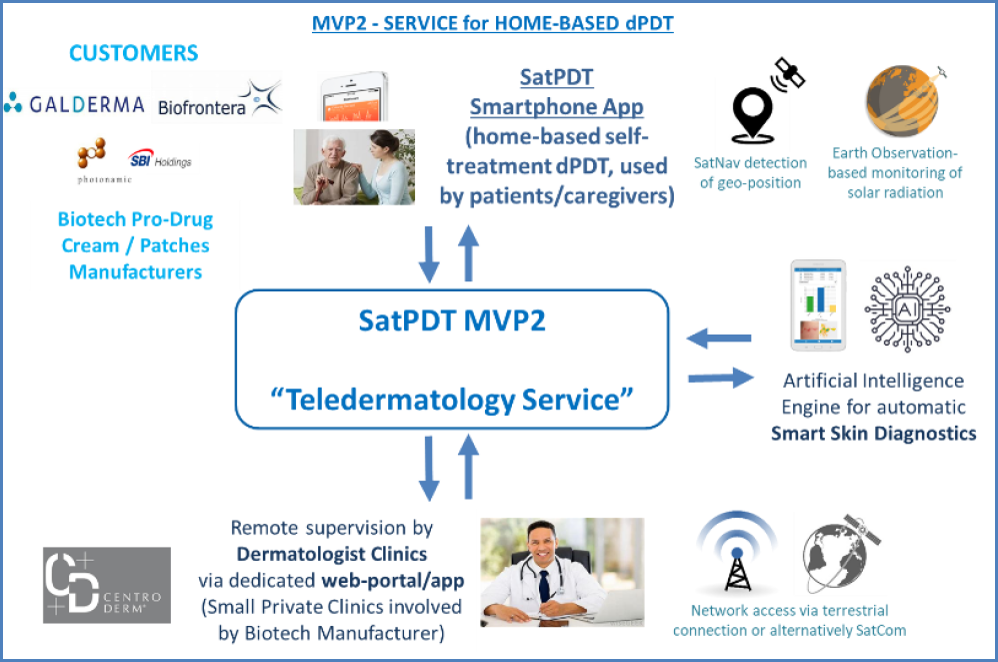
The second service, instead, is a home-based one to be directly used by the patients (or their caregivers). This is particularly intended for patients with relatively less severe AK (and able to use a mobile app) that could be confidently treated at home thanks to a remote supervision by clinical staff.
The service is provided through a mobile app that allows the patients to perform a “self-treatment”, i.e. to apply the biotech pro-drug product on the AK lesions by themselves and then to schedule & perform autonomously outdoor daylight exposure sessions (e.g. in their garden) by following the app guidance in real-time, based on Earth Observation satellite monitoring of solar irradiance (using SatNav geopositioning). In this case, the customers of the service are biotech pro-drug manufacturers involved through a B2B approach. The involvement of dermatologists (small private clinics) for the remote supervision is provided by the biotech pro-drug manufacturer itself (as a “bundled” offer). Also in this case, an Artificial Intelligence-based image processing component for smartphone-based AK diagnostics is included in the app for supporting the remote supervision (mainly for quantitively assessing the benefits of dPDT session on AK lesions).
Space Added Value
The SatPDT system relies on the following two space assets:
-
Earth Observation;
-
Satellite Navigation.
Earth Observation data are used for the near real-time monitoring of the dPDT therapy-effective solar irradiance at ground level with worldwide coverage. The same EO data are also used in addition to NWP data for the forecast of dPDT-effective solar irradiance (more accurate characterization of the forecasted atmospheric radiative transfer).
The satellite-based dosimetry aspect is a key and unprecedented added value provided by SatPDT. Indeed, the current alternative technologies for dPDT irradiance monitoring are less convenient for clinicians:
-
spectroradiometers, slightly more accurate than siHealth’s satellite-based patented method (McLellan et al., 2020) but extremely expensive and to be periodically calibrated (difficult to be maintained, needing expert personnel);
-
portable radiometers, not reliable and not accurate enough as well as not providing PDT-effective irradiance;
-
online weather forecasts, not accurate enough.
Finally, the real-time monitoring of the solar irradiance incident on a patient’s skin cancer/pre-cancer lesion is possible only if the location of the patient is accurately known. This will be possible thanks to SatNav technology, i.e. smartphone’s antenna for GPS and GNSS.
Current Status
-
product fully developed and validated, including a web-portal for clinicians (“SmartPDT-D”) and a mobile app for patients (“SmartPDT-P”)
-
73 daylight PDT treatment sessions were performed with SmartPDT by 4 clinics in 3 countries (UK, Spain, Germany) during the period July – September 2023 in the frame of the pilot demonstration activities: all session were successful, providing positive clinical outcomes and minimal or no side effects
-
the product is fully GDPR-compliant and certified as medical device class 1 (CE marked)
-
the product developed in this project is commercially called “SmartPDT” and ready to enter into the market for supporting dermatology clinics in delivering safe and effective dPDT treatments of skin cancer/pre-cancer (e.g. Actinic Keratosis


